
These strong intermolecular forces make it difficult to break the molecule apart, therefore more energy is needed causing a high melting and boiling point. The London force is the immediate attraction of electrons from one atom to the positive nuclei of other surrounding atoms. London Dispersion forces exist because the electrons are always in motion, temporarily becoming more positive or negative. These charged ends are attracted to other opposite charged ends creating a very strong intermolecular force. molecule, a group of two or more atoms that form the smallest identifiable unit into which a pure substance can be divided and still retain the composition.
/caffeine-molecule--computer-artwork-showing-the-structure-of-a-molecule-of-the-alkaloid-stimulant-and-legal-drug-caffeine--caffeine-is-found-in-drinks-such-as-tea--coffee--and-fizzy-drinks--it-is-also-found-in-chocolate--536232214-59aeb9e622fa3a0011c0d2b9.jpg)
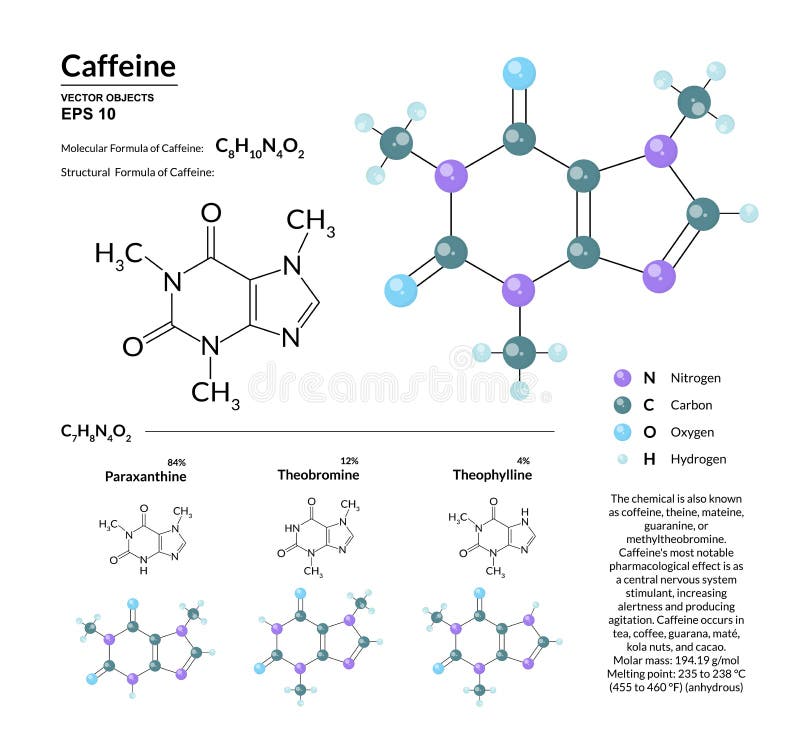
Pure caffeine (trimethylxanthine) occurs as a white powder or as silky needles, which melt at 238 C (460 F) it sublimes at 178 C (352 F) at atmospheric. Caffeine occurs in tea, coffee, guarana, mat, kola nuts, and cacao. These bond dipoles within the molecule create an overall molecular dipole therefore the molecule has one negative end and one positive end. caffeine, nitrogenous organic compound of the alkaloid group, substances that have marked physiological effects. They occur whenever there is a separation of positive and negative charges. Caffeine molecules have bond dipoles that measure the polarity of a chemical bond within a molecule. The intermolecular forces that are present in caffeine are London dispersion forces and Dipole-dipole forces.ĭipole-Dipole forces exist between the positive end of one polar molecule and the negative end of another polar molecule. Intermolecular forces also known as van der Waals forces consist of Hydrogen bonding, London dispersion forces and Dipole-dipole forces.


 0 kommentar(er)
0 kommentar(er)
